Expansive gene duplication and sub/neo-functionalization events are key factors driving the rapid divergence of terpenoid metabolism. These evolutionary events have been aided by the extensive functional plasticity of terpene synthases (TPS) and cytochrome P450 monooxygenases (P450), core enzymes in generating terpenoid chemical diversity, where minor active site alterations can dramatically impact product outcome, thus enabling the emergence of new functions with minimal investment in evolving new enzymes. Hence, there has been a long-standing interest in deciphering the mechanisms underlying TPS and P450 catalysis and substrate/product specificity. Combining protein crystallization and homology modeling with structure-guided site-directed mutagenesis approaches, we work to provide mechanistic insight into the catalytic specificity of TPS and P450 enzymes and apply this knowledge to protein engineering for advancing the synthetic biology platforms for terpenoid bioproduct manufacture.
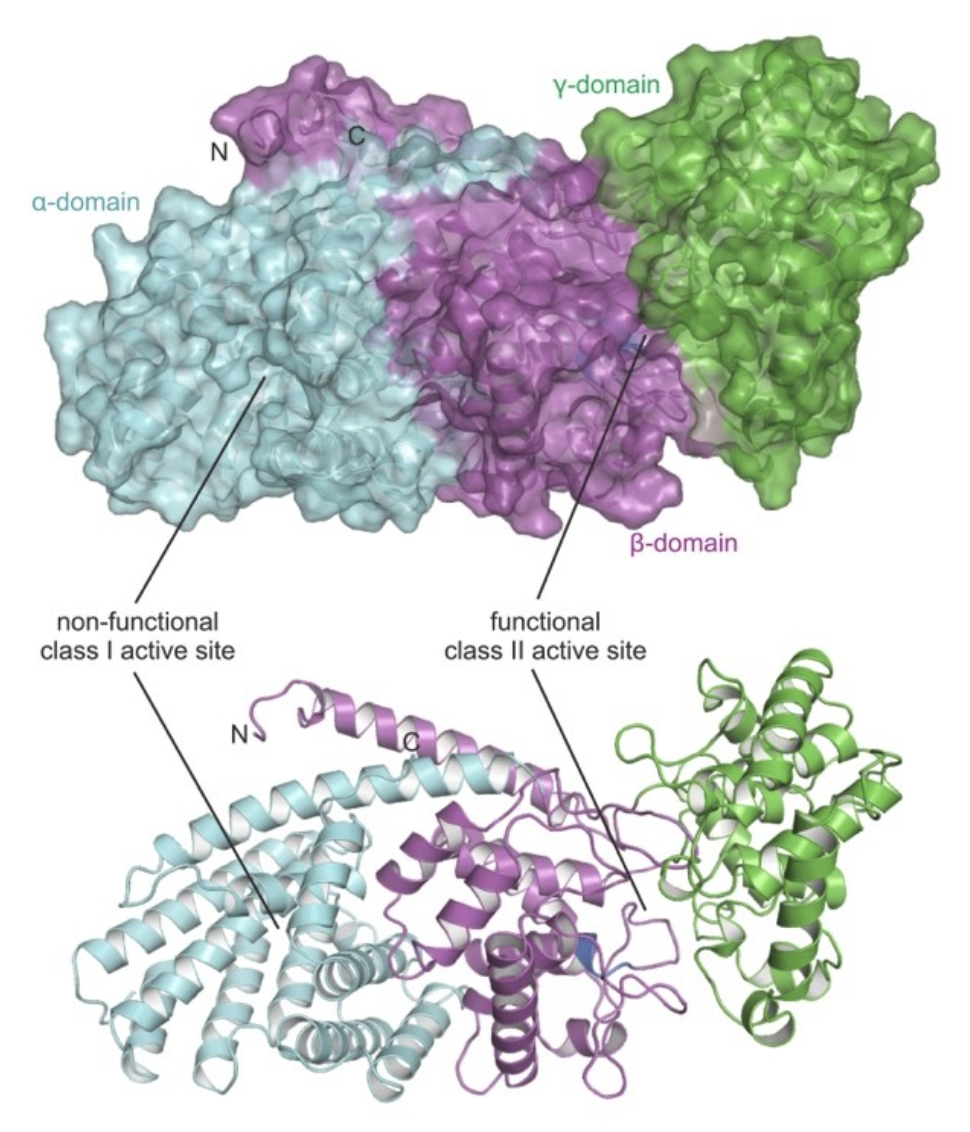
For example, we are investigating the biosynthesis of grindelic acid in gumweed (Grindelia spp.). Recently, we identified the crystal structure of Grindelia robusta 7,13-copalyl diphosphate synthase, GrTPS2, at 2.1 Å of resolution. GrTPS2 catalyzes the committed reaction in the biosynthesis of grindelic acid, which represents the signature metabolite in species of gumweed (Grindelia spp., Asteraceae). Grindelic acid has been explored as a potential source for drug leads and biofuel production. The GrTPS2 crystal structure adopts the conserved three-domain fold of class II diterpene synthases featuring a functional active site in the γβ-domain and a vestigial ɑ-domain. Substrate docking into the active site of the GrTPS2 apo protein structure predicted catalytic amino acids. Biochemical characterization of protein variants identified residues with impact on enzyme activity and catalytic specificity. Specifically, mutagenesis of Y457 provided mechanistic insight into the position-specific deprotonation of the intermediary carbocation to form the characteristic 7,13 double bond of 7,13-copalyl diphosphate.
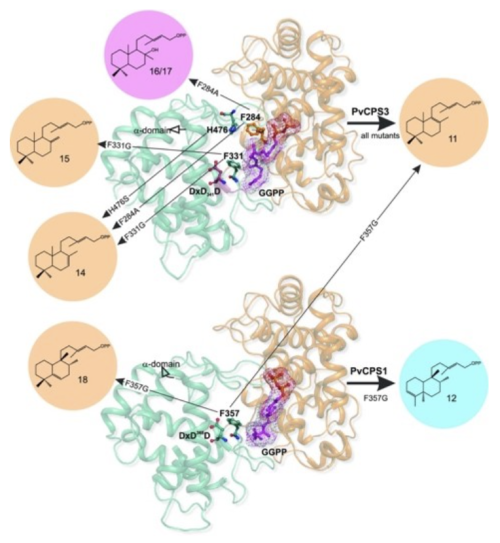
In another study, we investigated the catalytic mechanism of the diterpene synthase clerodienyl diphosphate synthase 1 (PvCPS1) from switchgrass (Panicum virgatum), which stereoselectively converts geranylgeranyl diphosphate (GGPP) into the clerodane diterpenoid, cis-trans-clerodienyl diphosphate (CLPP). Structure-guided point mutations of PvCPS1 redirected product stereoselectivity toward a range of related structures known as major catalytic outputs of other diterpene synthases, thus highlighting structural hotspots where even minor active site alterations would have resulted in the evolution of new products.
If you would like to read more:
- Cowie AE, Pereira JH, DeGiovanni A, McAndrew RP, Palayam M, Peek JO, Muchlinski AJ, Yoshikuni Y, Shabek N, Adams PD, Zerbe P (2024) The crystal structure of Grindelia robusta 7,13-copalyl diphosphate synthase reveals active site features controlling catalytic specificity. J Biol Chem 300:107921. [Link]
- Muchlinski A, Jia M, Tiedge K, Fell JS, Pelot KA, Chew L, Davisson D, Chen Y, Siegel J, Lovell JT, Zerbe P (2021) Cytochrome P450-catalyzed biosynthesis of furanoditerpenoids in the bioenergy crop switchgrass (Panicum virgatum L.). Plant J 108:1053-1068. [Link]
- Wang JZ, Lei Y, Xiao Y, He X, Liang J, Jiang J, Dong S, Ke H, Leon P, Zerbe P, Xiao Y, Dehesh K (2020) Uncovering the functional residues of Arabidopsis isoprenoid biosynthesis enzyme HDS. PNAS 117:355-361. [Link]
- Mafu S, Karunanithi PS, Palazzo TA, Harrod BL, Rodriguez SM, Mollhoff IN, O'Brien TE, Tong S, Fiehn O, Tantillo DJ, Bohlmann J, Zerbe P (2017) Biosynthesis of the microtubule-destabilizing diterpene pseudolaric acid B from golden larch involves an unusual diterpene synthase. PNAS 114:974-979. [Link]
- Pelot KA, Hagelthorn DM, Hong YJ, Tantillo DJ, Zerbe P (2018) Diterpene Synthase-Catalyzed Biosynthesis of Distinct Clerodane Stereoisomers. Chembiochem 20:111-117. [Link]
- Pelot KA, Mitchell R, Kwon M, Hagelthorn LM, Wardman JF, Chiang A, Bohlmann J, Ro DK, Zerbe P (2017) Biosynthesis of the psychotropic plant diterpene salvinorin A: Discovery and characterization of the Salvia divinorum clerodienyl diphosphate synthase. Plant J 89:885-897. [Link]
- Mafu S, Fischer E, Addison JB, Riberio Barbosana I, Zerbe P. Substitution of Two Active-Site Residues Alters C9-Hydroxylation in a Class II Diterpene Synthase. Chembiochem. 2016 Dec 14;17(24):2304-2307. doi: 10.1002/cbic.201600419. Epub 2016 Oct 31. PMID: 27735121. [Link]
This research has been generously supported by:

