To investigate and harness the vast chemical repertoire of plant terpenoid metabolism, a core focus of our research is the discovery of unusual and rare terpenoid-metabolic genes, enzymes, and their pathway products. We do this by combining genomics-enabled gene discovery, rapid enzyme biochemical characterization through microbial and plant co-expression assays, and de novo identification of novel metabolites using state-of-the-art mass spectrometry and NMR approaches. We have identified more than 150 of functionally distinct terpene synthase (TPS) and cytochrome P450 monooxygenase (P450) enzymes that catalyze the key reactions in generating terpenoid chemical diversity. We integrate these biochemical insights with in planta terpenoid profiling via GC- and LC-MS analyses, genetic gene function studies, as well as bioactivity analysis to assess the potential of select terpenoid metabolites for agricultural and other biotechnology applications.
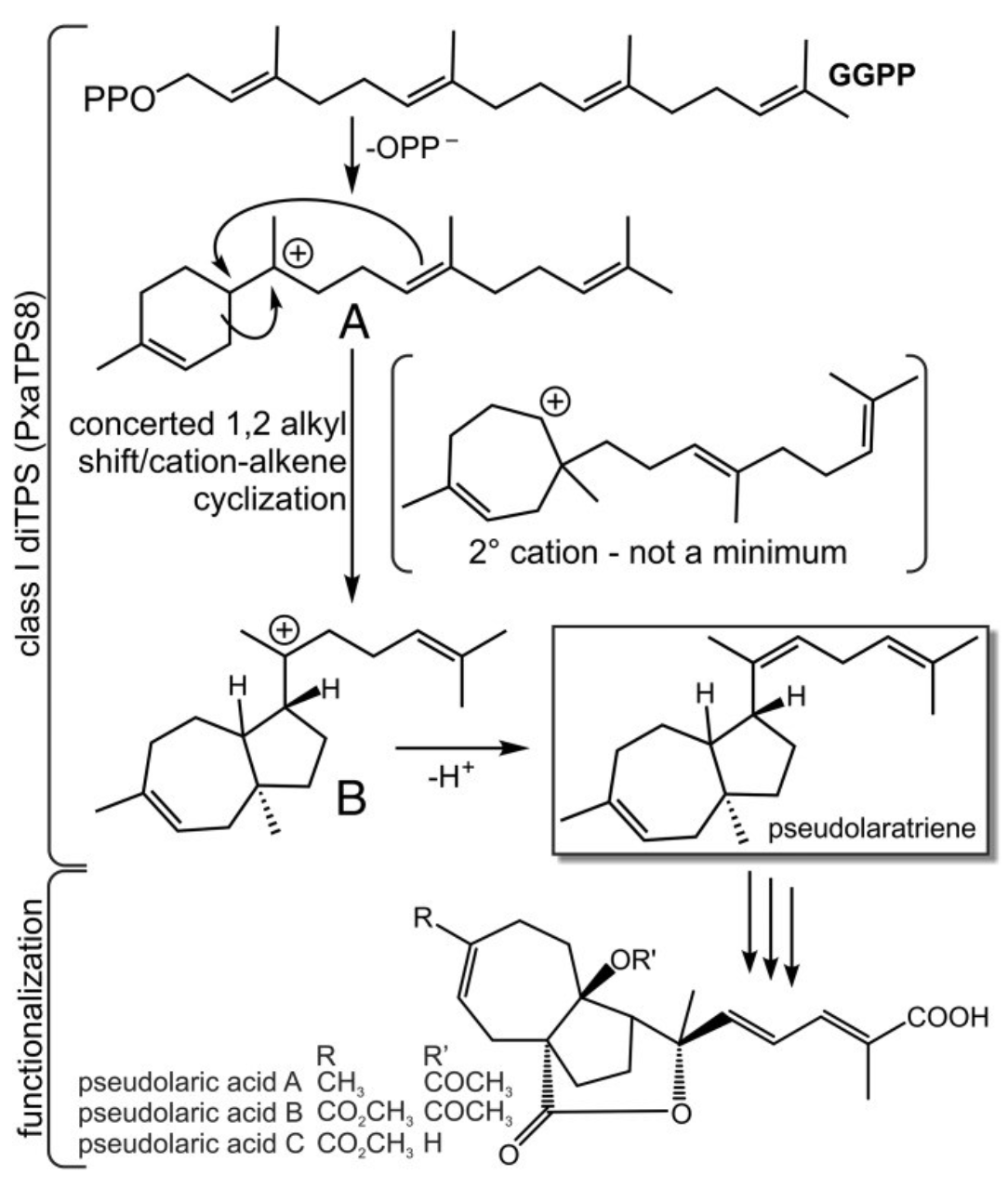
For example, we are investigating the biosynthesis of the diterpenoid, pseudolaric acid B (PAB), a potential chemotherapeutic agent from Golden larch (Pseudolarix amabilis). PAB is found, perhaps uniquely, in the coniferous tree golden larch (Pseudolarix amabilis, Pxa). We identified a golden larch terpene synthase 8 (PxaTPS8), an unusual diterpene synthase (diTPS) that catalyzes the first committed step in PAB biosynthesis. Mining of the golden larch root transcriptome revealed a large TPS family, including the monofunctional class I diTPS PxaTPS8, which converts geranylgeranyl diphosphate into a previously unknown 5,7-fused bicyclic diterpene, coined "pseudolaratriene." Combined NMR and quantum chemical analysis verified the structure of pseudolaratriene, and co-occurrence with PxaTPS8 and PAB in P amabilis tissues supports the intermediacy of pseudolaratriene in PAB metabolism. Although PxaTPS8 adopts the typical three-domain structure of diTPSs, sequence phylogeny places the enzyme with two-domain TPSs of mono- and sesqui-terpene biosynthesis. Site-directed mutagenesis of PxaTPS8 revealed several catalytic residues that, together with quantum chemical calculations, suggested a substantial divergence of PxaTPS8 from other TPSs leading to a distinct carbocation-driven reaction mechanism en route to the 5,7-trans-fused bicyclic pseudolaratriene scaffold. PxaTPS8 expression in microbial and plant hosts provided proof of concept for metabolic engineering of pseudolaratriene.
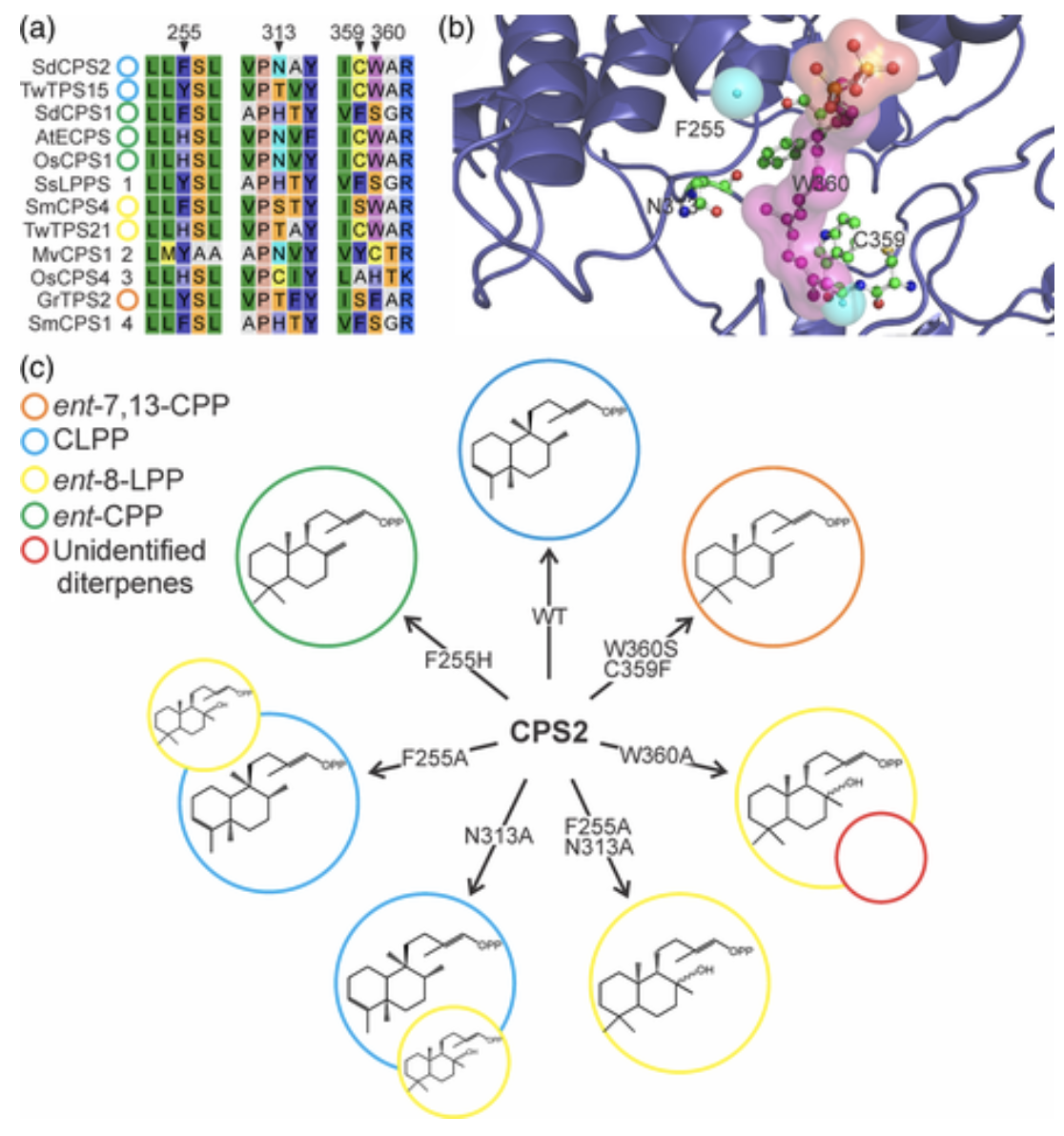
In another study, we investigated the biosynthesis of the clerodane-type diterpenoid, salvinorin A, from Salvia divinorum. Salvinorin A the main bioactive metabolite in S. divinorum and has attracted much interested due to its relevance as a non-nitrogenous natural compound known to function as an opioid-receptor agonist, thus having potential use in treating neuropsychiatric diseases and drug addictions. We discovered two S. divinorum diterpene synthases (diTPSs), the ent-copalyl diphosphate (ent-CPP) synthase SdCPS1, and the clerodienyl diphosphate (CLPP) synthase SdCPS2. Mining of leaf- and trichome-specific transcriptomes revealed five diTPSs, two of which are class II diTPSs (SdCPS1-2) and three are class I enzymes (SdKSL1-3). Of the class II diTPSs, transient expression in Nicotiana benthamiana identified SdCPS1 as an ent-CPP synthase, which is prevalent in roots and, together with SdKSL1, exhibits a possible dual role in general and specialized metabolism. In vivo co-expression and in vitro assays combined with nuclear magnetic resonance (NMR) analysis identified SdCPS2 as a CLPP synthase. A role of SdCPS2 in catalyzing the committed step in salvinorin A biosynthesis is supported by its biochemical function, trichome-specific expression and absence of additional class II diTPSs in S. divinorum. Structure-guided mutagenesis revealed four catalytic residues that enabled the re-programming of SdCPS2 activity to afford four distinct products, thus advancing our understanding of how neo-functionalization events have shaped the array of different class II diTPS functions in plants, and may promote synthetic biology platforms for a broader spectrum of diterpenoid bioproducts.
If you would like to read more:
- Zerbe P (2024) Plants against cancer: towards green Taxol production through pathway discovery and metabolic engineering. aBIOTECH 5:394-402. [Link]
- Karunanithi PS, Dhanota P, Addison JB, Tong S, Fiehn O, Zerbe P (2019) Functional characterization of the cytochrome P450 monooxygenase CYP71AU87 indicates a role in marrubiin biosynthesis in the medicinal plant Marrubium vulgare. BMC Plant Biol 19:114. [Link]
- Pelot KA, Hagelthorn LM, Addison JB, Zerbe P (2017) Biosynthesis of the oxygenated diterpene nezukol in the medicinal plant Isodon rubescens is catalyzed by a pair of diterpene synthases. PLoS One 12:e0176507. [Link]
- Mafu S, Karunanithi PS, Palazzo TA, Harrod BL, Rodriguez SM, Mollhoff IN, O'Brien TE, Tong S, Fiehn O, Tantillo DJ, Bohlmann J, Zerbe P (2017) Biosynthesis of the microtubule-destabilizing diterpene pseudolaric acid B from golden larch involves an unusual diterpene synthase. PNAS 114:974-979. [Link]
- Pelot KA, Mitchell R, Kwon M, Hagelthorn LM, Wardman JF, Chiang A, Bohlmann J, Ro DK, Zerbe P (2017) Biosynthesis of the psychotropic plant diterpene salvinorin A: Discovery and characterization of the Salvia divinorum clerodienyl diphosphate synthase. Plant J 89:885-897. [Link]
- Zerbe P, Rodriguez SM, Mafu S, Chiang A, Sandhu HK, O'Neil-Johnson M, Starks CM, Bohlmann J (2015) Exploring diterpene metabolism in non-model species: transcriptome-enabled discovery and functional characterization of labda-7,13E-dienyl diphosphate synthase from Grindelia robusta. Plant J 83:783-93. [Link]
This research has been generously supported by:

